MADAD
MADAD dedicated app is keeping all sort of emergency solutions at the single platform.
Translate
Sunday, 6 July 2025
Saturday, 5 July 2025
Monday, 21 February 2022
HUMAN Brain Anatomy
What is the brain?
The brain is a complex organ that controls thought, memory,
emotion, touch, motor skills, vision, breathing, temperature, hunger and every
process that regulates our body. Together, the brain and spinal cord that
extends from it make up the central nervous system, or CNS.
What
is the brain made of?
Weighing about 3 pounds in the average adult, the brain is about
60% fat. The remaining 40% is a combination of water, protein, carbohydrates and
salts. The brain itself is not a muscle.
It contains blood vessels and nerves, including neurons and glial cells.
What is the gray matter and white
matter?
Gray and
white matter are two different regions of the central nervous system. In the
brain, gray matter refers to the darker, outer portion, while white matter describes
the lighter, inner section underneath. In the spinal cord, this order is
reversed:
The white matter is on the outside, and the
gray matter sits within.
Gray matter is primarily
composed of neuron somas (the round central cell bodies), and white matter is
mostly made of axons (the long stems that connects neurons together) wrapped in
myelin (a protective coating). The different composition of neuron parts is why
the two appear as separate shades on certain scans
Motor and
sensory regions of the brain
Each region serves a
different role. Gray matter is primarily responsible for processing and
interpreting information, while white matter transmits that information to
other parts of the nervous system.
How does the brain work?
The brain sends and receives chemical and electrical signals
throughout the body. Different signals control different processes, and your
brain interprets each. Some make you feel tired, for example, while others make
you feel pain.
Some messages are kept within the brain, while others are relayed
through the spine and across the body’s vast network of nerves to distant extremities.
To do this, the central nervous system relies on billions of neurons (nerve
cells).
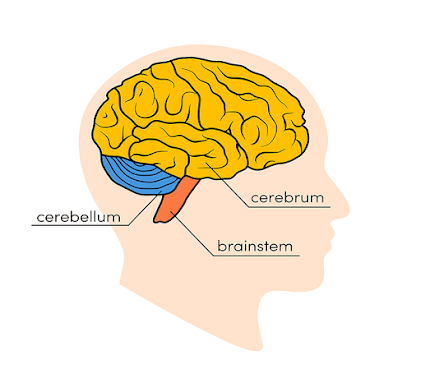
Main
Parts of the Brain and Their Functions
At a high level, the brain can be divided into the cerebrum,
brainstem and cerebellum.
Cerebrum
The
cerebrum (front of brain) comprises gray matter (the cerebral cortex) and white
matter at its center. The largest part of the brain, the cerebrum initiates and
coordinates movement and regulates temperature. Other areas of the cerebrum
enable speech, judgment, thinking and reasoning, problem-solving, emotions and
learning. Other functions relate to vision, hearing, touch and other senses.
Cerebral Cortex
Cortex
is Latin for “bark,” and describes the outer gray matter covering of the
cerebrum. The cortex has a large surface area due to its folds, and comprises
about half of the brain’s weight.
The
cerebral cortex is divided into two halves, or hemispheres. It is covered with
ridges (gyri) and folds (sulci). The two halves join at a large, deep sulcus
(the interhemispheric fissure, AKA the medial longitudinal fissure) that runs
from the front of the head to the back. The right hemisphere controls the left
side of the body, and the left half controls the right side of the body. The
two halves communicate with one another through a large, C-shaped structure of
white matter and nerve pathways called the corpus callosum. The corpus callosum
is in the center of the cerebrum.
Brainstem
The
brainstem (middle of brain) connects the cerebrum with the spinal cord. The
brainstem includes the midbrain, the pons and the medulla.
- Midbrain. The
midbrain (or mesencephalon) is a very complex structure with a range of
different neuron clusters (nuclei and colliculi), neural pathways and
other structures. These features facilitate various functions, from
hearing and movement to calculating responses and environmental changes.
The midbrain also contains the substantia nigra, an area affected by
Parkinson’s disease that is rich in dopamine neurons and part of the basal
ganglia, which enables movement and coordination.
- Pons. The pons is the
origin for four of the 12 cranial nerves, which enable a range of
activities such as tear production, chewing, blinking, focusing vision,
balance, hearing and facial expression. Named for the Latin word for
“bridge,” the pons is the connection between the midbrain and the medulla.
- Medulla. At the bottom of
the brainstem, the medulla is where the brain meets the spinal cord. The
medulla is essential to survival. Functions of the medulla regulate many
bodily activities, including heart rhythm, breathing, blood flow, and
oxygen and carbon dioxide levels. The medulla produces reflexive
activities such as sneezing, vomiting, coughing and swallowing.
The spinal cord extends from the
bottom of the medulla and through a large opening in the bottom of the skull.
Supported by the vertebrae, the spinal cord carries messages to and from the
brain and the rest of the body.
Cerebellum
The
cerebellum (“little brain”) is a fist-sized portion of the brain located at the
back of the head, below the temporal and occipital lobes and above the
brainstem. Like the cerebral cortex, it has two hemispheres. The outer portion
contains neurons, and the inner area communicates with the cerebral cortex. Its
function is to coordinate voluntary muscle movements and to maintain posture,
balance and equilibrium. New studies are exploring the cerebellum’s roles in
thought, emotions and social behavior, as well as its possible involvement in
addiction, autism and schizophrenia.
Brain Coverings: Meninges
Three layers of protective covering called meninges surround the brain and the spinal cord.
- The outermost
layer, the dura mater, is thick and
tough. It includes two layers: The periosteal layer of the dura mater
lines the inner dome of the skull (cranium) and the meningeal layer is
below that. Spaces between the layers allow for the passage of veins and
arteries that supply blood flow to the brain.
- The arachnoid mater is a thin,
weblike layer of connective tissue that does not contain nerves or blood
vessels. Below the arachnoid mater is the cerebrospinal fluid, or CSF.
This fluid cushions the entire central nervous system (brain and spinal
cord) and continually circulates around these structures to remove
impurities.
- The pia mater is a thin
membrane that hugs the surface of the brain and follows its contours. The
pia mater is rich with veins and arteries.
Lobes of the Brain and What They Control
Each
brain hemisphere (parts of the cerebrum) has four sections, called lobes:
frontal, parietal, temporal and occipital. Each lobe controls specific
functions.
- Frontal lobe. The largest lobe of the brain,
located in the front of the head, the frontal lobe is involved in
personality characteristics, decision-making and movement. Recognition of
smell usually involves parts of the frontal lobe. The frontal lobe
contains Broca’s area, which is associated with speech ability.
- Parietal lobe. The middle part
of the brain, the parietal lobe helps a person identify objects and
understand spatial relationships (where one’s body is compared with
objects around the person). The parietal lobe is also involved in
interpreting pain and touch in the body. The parietal lobe houses
Wernicke’s area, which helps the brain understand spoken language.
- Occipital lobe. The
occipital lobe is the back part of the brain that is involved with vision.
- Temporal lobe. The sides
of the brain, temporal lobes are involved in short-term memory, speech,
musical rhythm and some degree of smell recognition.
Deeper
Structures Within the Brain
Pituitary Gland
Sometimes
called the “master gland,” the pituitary gland is a pea-sized structure found
deep in the brain behind the bridge of the nose. The pituitary gland governs
the function of other glands in the body, regulating the flow of hormones from
the thyroid, adrenals, ovaries and testicles. It receives chemical signals from
the hypothalamus through its stalk and blood supply.
Hypothalamus
The
hypothalamus is located above the pituitary gland and sends it chemical
messages that control its function. It regulates body temperature, synchronizes
sleep patterns, controls hunger and thirst and also plays a role in some
aspects of memory and emotion.
Amygdala
Small,
almond-shaped structures, an amygdala is located under each half (hemisphere)
of the brain. Included in the limbic system, the amygdalae regulate emotion and
memory and are associated with the brain’s reward system, stress, and the
“fight or flight” response when someone perceives a threat.
Hippocampus
A
curved seahorse-shaped organ on the underside of each temporal lobe, the
hippocampus is part of a larger structure called the hippocampal formation. It
supports memory, learning, navigation and perception of space. It receives
information from the cerebral cortex and may play a role in Alzheimer’s
disease.
Pineal Gland
The
pineal gland is located deep in the brain and attached by a stalk to the top of
the third ventricle. The pineal gland responds to light and dark and secretes
melatonin, which regulates circadian rhythms and the sleep-wake cycle.
Ventricles and Cerebrospinal Fluid
Deep
in the brain are four open areas with passageways between them. They also open
into the central spinal canal and the area beneath arachnoid layer of the
meninges.
The
ventricles manufacture cerebrospinal fluid, or CSF, a watery fluid that circulates in and around the
ventricles and the spinal cord, and between the meninges. CSF surrounds and
cushions the spinal cord and brain, washes out waste and impurities, and
delivers nutrients.
Blood Supply to the Brain
Two
sets of blood vessels supply blood and oxygen to the brain: the vertebral arteries and the carotid arteries.
The
external carotid arteries extend up the sides of your neck, and are where you
can feel your pulse when you touch the area with your fingertips. The internal
carotid arteries branch into the skull and circulate blood to the front part of
the brain.
The
vertebral arteries follow the spinal column into the skull, where they join
together at the brainstem and form the basilar artery, which supplies blood to the rear portions of the brain.
The circle of Willis, a loop of blood vessels
near the bottom of the brain that connects major arteries, circulates blood
from the front of the brain to the back and helps the arterial systems
communicate with one another.
Cranial Nerves
Inside
the cranium (the dome of the skull), there are 12 nerves, called cranial
nerves:
- Cranial nerve 1:
The first is the olfactory nerve, which allows for your sense of
smell.
- Cranial nerve 2:
The optic nerve governs
eyesight.
- Cranial nerve 3:
The oculomotor nerve controls
pupil response and other motions of the eye, and branches out from the
area in the brainstem where the midbrain meets the pons.
- Cranial nerve 4:
The trochlear nerve controls
muscles in the eye. It emerges from the back of the midbrain part of the
brainstem.
- Cranial nerve 5:
The trigeminal nerve is the
largest and most complex of the cranial nerves, with both sensory and
motor function. It originates from the pons and conveys sensation from the
scalp, teeth, jaw, sinuses, parts of the mouth and face to the brain,
allows the function of chewing muscles, and much more.
- Cranial nerve 6:
The abducens nerve innervates
some of the muscles in the eye.
- Cranial nerve 7:
The facial nerve supports
face movement, taste, glandular and other functions.
- Cranial nerve 8:
The vestibulocochlear nerve facilitates
balance and hearing.
- Cranial nerve 9:
The glossopharyngeal nerve allows
taste, ear and throat movement, and has many more functions.
- Cranial nerve
10: The vagus nerve allows
sensation around the ear and the digestive system and controls motor
activity in the heart, throat and digestive system.
- Cranial nerve
11: The accessory nerve innervates
specific muscles in the head, neck and shoulder.
- Cranial nerve
12: The hypoglossal nerve supplies
motor activity to the tongue.
The
first two nerves originate in the cerebrum, and the remaining 10 cranial nerves
emerge from the brainstem, which has three parts: the midbrain, the pons and
the medulla.
Thursday, 11 November 2021
Cancer Therapy by Immune checkpoint
Cancer kills millions of people every year and is one of humanity’s greatest health challenges. By stimulating the inherent ability of our immune system to attack tumor cells 2018 Nobel Laureates James P. Allison and Tasuku Honjo have established an entirely new principle for cancer therapy.
James P. Allison studied a known protein that
functions as a brake on the immune system. He realized the potential of
releasing the brake and thereby unleashing our immune cells to attack tumors.
He then developed this concept into a brand new approach for treating patients.
In parallel, Tasuku Honjo discovered a
protein on immune cells and, after careful exploration of its function,
eventually revealed that it also operates as a brake, but with a different
mechanism of action. Therapies based on his discovery proved to be strikingly
effective in the fight against cancer.
Allison and Honjo showed how different
strategies for inhibiting the brakes on the immune system can be used in the
treatment of cancer. The seminal discoveries by the two Laureates constitute a
landmark in our fight against cancer.
Cancer
comprises many different diseases, all characterized by uncontrolled
proliferation of abnormal cells with capacity for spread to healthy organs and
tissues. A number of therapeutic approaches are available for cancer treatment,
including surgery, radiation, and other strategies, some of which have been
awarded previous Nobel Prizes. These include methods for hormone treatment for
prostate cancer (Huggins, 1966), chemotherapy (Elion and Hitchings, 1988), and
bone marrow transplantation for leukemia (Thomas 1990). However, advanced
cancer remains immensely difficult to treat, and novel therapeutic strategies
are desperately needed.
In the late 19th century and beginning of the
20th century the concept emerged that activation of the immune system might be
a strategy for attacking tumor cells. Attempts were made to infect patients
with bacteria to activate the defense. These efforts only had modest effects,
but a variant of this strategy is used today in the treatment of bladder
cancer. It was realized that more knowledge was needed. Many scientists engaged
in intense basic research and uncovered fundamental mechanisms regulating
immunity and also showed how the immune system can recognize cancer cells.
Despite remarkable scientific progress, attempts to develop generalizable new
strategies against cancer proved difficult.
Accelerators
and brakes in our immune system
The
fundamental property of our immune system is the ability to discriminate “self”
from “non-self” so that invading bacteria, viruses and other dangers can be
attacked and eliminated. T cells, a type of white blood cell, are key players
in this defense. T cells were shown to have receptors that bind to structures
recognized as non-self and such interactions trigger the immune system to
engage in defense. But additional proteins acting as T-cell accelerators are
also required to trigger a full-blown immune response. Many scientists
contributed to this important basic research and identified other proteins that
function as brakes on the T cells, inhibiting immune activation. This intricate
balance between accelerators and brakes is essential for tight control. It
ensures that the immune system is sufficiently engaged in attack against
foreign microorganisms while avoiding the excessive activation that can lead to
autoimmune destruction of healthy cells and tissues.
A
new principle for immune therapy
During
the 1990s, in his laboratory at the University of California, Berkeley, James
P. Allison studied the T-cell protein CTLA-4. He was one of several scientists
who had made the observation that CTLA-4 functions as a brake on T cells. Other
research teams exploited the mechanism as a target in the treatment of
autoimmune disease. Allison, however, had an entirely different idea. He had
already developed an antibody that could bind to CTLA-4 and block its function .
He now set out to investigate if CTLA-4 blockade could disengage the T-cell
brake and unleash the immune system to attack cancer cells.
The results were spectacular.
Mice with cancer had been cured by treatment with the antibodies that inhibit
the brake and unlock antitumor T-cell activity. Despite little interest from
the pharmaceutical industry, Allison continued his intense efforts to develop
the strategy into a therapy for humans. Promising results soon emerged from
several groups, and in 2010 an important clinical study showed striking effects
in patients with advanced melanoma, a type of skin cancer. In several patients
signs of remaining cancer disappeared. Such remarkable results had never been
seen before in this patient group.
Discovery
of PD-1 and its importance for cancer therapy
In
1992, a few years before Allison’s discovery, Tasuku Honjo discovered PD-1,
another protein expressed on the surface of T-cells. Determined to unravel its
role, he meticulously explored its function in a series of elegant experiments
performed over many years in his laboratory at Kyoto University. The results
showed that PD-1, similar to CTLA-4, functions as a T-cell brake, but operates
by a different mechanism. In animal experiments, PD-1 blockade was also shown
to be a promising strategy in the fight against cancer, as demonstrated by
Honjo and other groups. This paved the way for utilizing PD-1 as a target in
the treatment of patients. Clinical development ensued, and in 2012 a key study
demonstrated clear efficacy in the treatment of patients with different types
of cancer. Results were dramatic, leading to long-term remission and possible
cure in several patients with metastatic cancer, a condition that had
previously been considered essentially untreatable.
Immune
checkpoint therapy for cancer today and in the future
After
the initial studies showing the effects of CTLA-4 and PD-1 blockade, the
clinical development has been dramatic. We now know that the treatment, often
referred to as “immune checkpoint therapy”, has fundamentally changed the
outcome for certain groups of patients with advanced cancer. Similar to other
cancer therapies, adverse side effects are seen, which can be serious and even
life threatening. They are caused by an overactive immune response leading to
autoimmune reactions, but are usually manageable. Intense continuing research
is focused on elucidating mechanisms of action, with the aim of improving
therapies and reducing side effects.
Of
the two treatment strategies, checkpoint therapy against PD-1 has proven more
effective and positive results are being observed in several types of cancer,
including lung cancer, renal cancer, lymphoma and melanoma. New clinical
studies indicate that combination therapy, targeting both CTLA-4 and PD-1, can
be even more effective, as demonstrated in patients with melanoma. Thus,
Allison and Honjo have inspired efforts to combine different strategies to
release the brakes on the immune system with the aim of eliminating tumor cells
even more efficiently. A large number of checkpoint therapy trials are
currently underway against most types of cancer, and new checkpoint proteins
are being tested as targets.
Figure: Upper left: Activation of T cells
requires that the T-cell receptor binds to structures on other immune cells
recognized as ”non-self”. A protein functioning as a T-cell accelerator is also
required for T cell activation. CTLA- 4 functions as a brake on T cells that
inhibits the function of the accelerator. Lower left: Antibodies (green) against CTLA-4 block the function of the
brake leading to activation of T cells and attack on cancer cells.Upper
right: PD-1 is another T-cell brake that inhibits T-cell activation. Lower right: Antibodies against PD-1 inhibit the function of the brake
leading to activation of T cells and highly efficient attack on cancer cells.
James P. Allison was born
1948 in Alice, Texas, USA. He received his PhD in 1973 at the University of
Texas, Austin. From 1974-1977 he was a postdoctoral fellow at the Scripps
Clinic and Research Foundation, La Jolla, California. From 1977-1984 he was a
faculty member at University of Texas System Cancer Center, Smithville, Texas;
from 1985-2004 at University of California, Berkeley and from 2004-2012 at
Memorial Sloan-Kettering Cancer Center, New York. From 1997-2012 he was an
Investigator at the Howard Hughes Medical Institute. Since 2012 he has been
Professor at University of Texas MD Anderson Cancer Center, Houston, Texas and
is affiliated with the Parker Institute for Cancer Immunotherapy.
Tasuku Honjo was born in
1942 in Kyoto, Japan. In 1966 he became an MD, and from 1971-1974 he was a
research fellow in USA at Carnegie Institution of Washington, Baltimore and at
the National Institutes of Health, Bethesda, Maryland. He received his PhD in
1975 at Kyoto University. From 1974-1979 he was a faculty member at Tokyo
University and from 1979-1984 at Osaka University. Since 1984 he has been
Professor at Kyoto University. He was a Faculty Dean from 1996-2000 and from
2002-2004 at Kyoto University.
Monday, 8 November 2021
How Oxygen levels affect cellular metabolism
Animals
need oxygen for the conversion of food into useful energy. The fundamental
importance of oxygen has been understood for centuries, but how cells adapt to
changes in levels of oxygen has long been unknown.
William
G. Kaelin Jr., Sir Peter J. Ratcliffe and Gregg L. Semenza discovered how cells
can sense and adapt to changing oxygen availability. They identified molecular
machinery that regulates the activity of genes in response to varying levels of
oxygen.
The
seminal discoveries by 2019 Nobel Laureates revealed the mechanism for one of
life’s most essential adaptive processes. They established the basis for our
understanding of how oxygen levels affect cellular metabolism and physiological
function. Their discoveries have also paved the way for promising new
strategies to fight anemia, cancer and many other diseases.
Oxygen
at center stage
Oxygen,
with the formula O2, makes up about one fifth of Earth’s
atmosphere. Oxygen is essential for animal life: it is used by the mitochondria
present in virtually all animal cells in order to convert food into useful
energy. Otto Warburg,
the recipient of the 1931 Nobel Prize in Physiology or Medicine, revealed that
this conversion is an enzymatic process.
During
evolution, mechanisms developed to ensure a sufficient supply of oxygen to
tissues and cells. The carotid body, adjacent to large blood vessels on both
sides of the neck, contains specialized cells that sense the blood’s oxygen
levels. The 1938 Nobel Prize in Physiology or Medicine to Corneille
Heymans awarded discoveries showing how blood oxygen sensing
via the carotid body controls our respiratory rate by communicating directly
with the brain.
HIF
(Hypoxia Inducible Factor)
In
addition to the carotid body-controlled rapid adaptation to low oxygen levels (hypoxia), there are other fundamental
physiological adaptations. A key physiological response to hypoxia is the rise
in levels of the hormone erythropoietin (EPO), which leads to increased
production of red blood cells (erythropoiesis). The importance of hormonal
control of erythropoiesis was already known at the beginning of the 20th century,
but how this process was itself controlled by O2 remained a mystery.
Gregg
Semenza studied the EPO gene and how it is regulated by varying oxygen levels.
By using gene-modified mice, specific DNA segments located next to the EPO gene
were shown to mediate the response to hypoxia. Sir Peter Ratcliffe also studied
O2-dependent regulation of the EPO gene, and
both research groups found that the oxygen sensing mechanism was present in
virtually all tissues, not only in the kidney cells where EPO is normally produced.
These were important findings showing that the mechanism was general and
functional in many different cell types.
Semenza
wished to identify the cellular components mediating this response. In cultured
liver cells he discovered a protein complex that binds to the identified DNA
segment in an oxygen-dependent manner. He called this complex the hypoxia-inducible factor (HIF) . Extensive
efforts to purify the HIF complex began, and in 1995, Semenza was able to
publish some of his key findings, including identification of the genes
encoding HIF. HIF was found to consist of two different DNA-binding proteins,
so called transcription factors, now named HIF-1α and ARNT. Now the researchers
could begin solving the puzzle, allowing them to understand which additional
components were involved and how the machinery works.
Erythropoietin
(EPO)
Erythropoietin
(EPO) is a hormone produced primarily by the kidneys, with small amounts made
by the liver. EPO plays a key role in the production of red blood cells (RBCs),
which carry oxygen from the lungs to the rest of the body.
The body uses a dynamic feedback system
to help maintain sufficient oxygen levels and a relatively stable number of
RBCs in the blood.
·
Erythropoietin
is produced and released into the blood by the kidneys in response to low blood
oxygen levels (hypoxemia). The amount of erythropoietin released depends on how
low the oxygen level is and the ability of the kidneys to produce
erythropoietin.
·
EPO
is carried to the bone marrow, where it stimulates production of red blood
cells. The hormone is active for a short period of time and then eliminated
from the body in the urine.
·
As
oxygen levels in the blood rise to normal or near normal levels, the kidneys
slow production of EPO.
However,
if your kidneys are damaged and do not produce enough erythropoietin, then too
few RBCs are produced and you can becomes anemic. Similarly, if your bone
marrow is unable to respond to the stimulation from EPO, then you may become
anemic. This can occur with some bone marrow
disorders or with chronic diseases, such as rheumatoid
arthritis.
If
you have a condition that affects the amount of oxygen you breathe in, such as
a lung disease,
you may produce more EPO to try to compensate for the low oxygen level. People
who live at high altitudes may also have higher levels of EPO and so do chronic
tobacco smokers.
If
you produce too much erythropoietin, which can happen with some benign or
malignant kidney tumors and with a variety of other cancers, you may produce
too many RBCs (polycythemia or erythrocytosis). This can lead to an
increase in the blood’s thickness (viscosity) and sometimes to high blood
pressure (hypertension),
blood clots (thrombosis), heart attack,
or stroke.
Rarely, polycythemia is caused by a bone marrow disorder called polycythemia
vera, not by increased erythropoietin.
VHL:
Von Hippel Lindau
When
oxygen levels are high, cells contain very little HIF-1α. However, when oxygen
levels are low, the amount of HIF-1α increases so that it can bind to and thus
regulate the EPO gene as well as other genes with HIF-binding DNA segments
(Figure 1). Several research groups showed that HIF-1α, which is normally
rapidly degraded, is protected from degradation in hypoxia. At normal oxygen
levels, a cellular machine called the proteasome, recognized by the 2004
Nobel Prize in Chemistry to Aaron
Ciechanover, Avram Hershko and Irwin Rose,
degrades HIF-1α. Under such conditions a small peptide, ubiquitin, is
added to the HIF-1α protein. Ubiquitin functions as a tag for proteins destined
for degradation in the proteasome. How ubiquitin binds to HIF-1α in an
oxygen-dependent manner remained a central question.
The
answer came from an unexpected direction. At about the same time as Semenza and
Ratcliffe were exploring the regulation of the EPO gene, cancer researcher
William Kaelin, Jr. was researching an inherited syndrome, von Hippel-Lindau’s
disease (VHL disease). This genetic disease leads to dramatically increased
risk of certain cancers in families with inherited VHL mutations. Kaelin showed
that the VHL gene encodes a protein that prevents the onset of cancer. Kaelin
also showed that cancer cells lacking a functional VHL gene express abnormally
high levels of hypoxia-regulated genes; but that when the VHL gene was
reintroduced into cancer cells, normal levels were restored. This was an
important clue showing that VHL was somehow involved in controlling responses
to hypoxia. Additional clues came from several research groups showing that VHL
is part of a complex that labels proteins with ubiquitin, marking them for
degradation in the proteasome. Ratcliffe and his research group then made a key
discovery: demonstrating that VHL can physically interact with HIF-1α and is
required for its degradation at normal oxygen levels. This conclusively linked
VHL to HIF-1α.
Under normal oxygen levels,
hydroxyl groups are added at two specific positions in HIF-1α . This protein
modification, called prolyl hydroxylation, allows VHL to recognize and bind to HIF-1α and thus
explained how normal oxygen levels control rapid HIF-1α degradation with the
help of oxygen-sensitive enzymes (so-called prolyl hydroxylases). Further research by
Ratcliffe and others identified the responsible prolyl hydroxylases. It was
also shown that the gene activating function of HIF-1α was regulated by
oxygen-dependent hydroxylation. The Nobel Laureates had now elucidated the
oxygen sensing mechanism and had shown how it works.
Figure 1. When oxygen levels are low (hypoxia), HIF-1α is
protected from degradation and accumulates in the nucleus, where it associates
with ARNT and binds to specific DNA sequences (HRE) in hypoxia-regulated genes
(1). At normal oxygen levels, HIF-1α is rapidly degraded by the proteasome (2).
Oxygen regulates the degradation process by the addition of hydroxyl groups
(OH) to HIF-1α (3). The VHL protein can then recognize and form a complex with
HIF-1α leading to its degradation in an oxygen-dependent manner (4).
Oxygen
shapes physiology and pathology
Thanks
to the groundbreaking work of these Nobel Laureates, we know much more about
how different oxygen levels regulate fundamental physiological processes.
Oxygen sensing allows cells to adapt their metabolism to low oxygen levels: for
example, in our muscles during intense exercise. Other examples of adaptive
processes controlled by oxygen sensing include the generation of new blood
vessels and the production of red blood cells. Our immune system and many other
physiological functions are also fine-tuned by the O2-sensing machinery. Oxygen
sensing has even been shown to be essential during fetal development for
controlling normal blood vessel formation and placenta development.
Oxygen
sensing is central to a large number of diseases . For example, patients with
chronic renal failure often suffer from severe anemia due to decreased EPO
expression. EPO is produced by cells in the kidney and is essential for
controlling the formation of red blood cells, as explained above. Moreover, the
oxygen-regulated machinery has an important role in cancer. In tumors, the
oxygen-regulated machinery is utilized to stimulate blood vessel formation and
reshape metabolism for effective proliferation of cancer cells. Intense ongoing
efforts in academic laboratories and pharmaceutical companies are now focused
on developing drugs that can interfere with different disease states by either
activating, or blocking, the oxygen-sensing machinery.
Figure 2. The awarded mechanism for oxygen sensing has fundamental importance in physiology, for example for our metabolism, immune response and ability to adapt to exercise. Many pathological processes are also affected. Intensive efforts are ongoing to develop new drugs that can either inhibit or activate the oxygen-regulated machinery for treatment of anemia, cancer and other diseases.
Von Hippel-Lindau Syndrome
What
is von Hippel-Lindau disease?
Von Hippel-Lindau syndrome (VHL) is a hereditary
condition associated with tumors arising in multiple organs. VHL-related tumors
include hemangioblastomas, which are blood vessel tumors of the brain, spinal
cord, and retina. The retinal tumors are also called retinal angiomas, which
can lead to blindness if not treated in a timely manner. People with VHL also
have an increased risk of developing clear cell renal cell carcinoma (ccRCC),
which is a specific type of kidney cancer, as well as a type of tumor in the
pancreas known as pancreatic
neuroendocrine tumor (pNET). Tumors of the adrenal gland or pheochromocytoma can
also develop, with a small number becoming metastatic, meaning they spread to
other parts of the body.
Other features of VHL include: kidney cysts, which are closed sacs usually
filled with fluid; pancreatic cysts, epididymal cystadenomas, which are tumors
near a man’s testicles; broad ligament cystadenomas, which occur near the
fallopian tubes in women; and endolymphatic sac tumors (ELST), which are tumors
of the inner ear that may cause hearing loss.
What causes VHL?
VHL is a
genetic condition. This means that the risk of developing certain types of
tumors and other features of VHL can be passed from generation to generation.
The gene associated with VHL is also called VHL. Inheriting a deletion or
mutation (alteration) in the VHL gene gives a person an increased risk of developing any of the different
signs of VHL explained above, called manifestations. Nearly everyone who has
VHL syndrome has an identifiable VHL genetic
mutation.
How is VHL inherited?
Normally, every
cell has 2 copies of each gene: 1 inherited from the mother and 1 inherited from
the father. VHL follows an autosomal dominant inheritance pattern, in which
inheriting 1 copy of the altered gene will likely result in a mutation of the
second (normal) copy of the gene. This puts the individual at risk for
developing cancer.
The increase in body size of humans and other vertebrates requires a
physiological
infrastructure to provide adequate delivery of oxygen to tissues
and cells to
maintain oxygen homeostasis. The heart, lungs and the vasculature
are all part
of a highly regulated system that ensures the distribution of the
precise
amount of oxygen needed throughout the mammalian organism.
1. The role of HIF-1
α pathway in cellular
adaptation to hypoxic stress
Mammalian cells need to maintain
proper oxygen hemostasis in order to execute their aerobic metabolism and
energy generation. In cancer, heart diseases, or chronic obstructive pulmonary
disorders, the cellular oxygen balance is highly impaired, and cells
become hypoxic (having low oxygen (O2) levels). Hypoxia is common in
many types of solid tumors, where tumor
cells proliferate rapidly and form large solid tumor masses, leading to obstruction
and compression of the blood vessels surrounding these masses. These abnormal
blood vessels often do not function properly and result in poor O2 supply
to the center tumor regions2.
Tumor cells in this hypoxic region begin to adapt these low oxygen tension
conditions by activating several survival pathways. Activation of HIF-1
transcription factor is the most recognized pathway adopted by hypoxic cells in
this harsh microenvironment
2. Regulation of HIF-1α pathway
The activity and accumulation of
HIF-1α protein
were found to be regulated at different levels throughout its life cycle inside
the cells. Independently from O2 levels, HIF-1α is constitutively
transcribed and synthesized through a series of signaling events involving
several growth factors and other signaling molecules. HIF-1α undergoes quick degradation
under normoxic conditions and normally has a very short half-life (about
5 min). In contrast, under hypoxic conditions, several pathways have been
shown to control HIF-1α stability
and transcriptional activity via post-transnational modifications involving
hydroxylation, acetylation, ubiquitination, and phosphorylation reactions